Especially we look at the reaction paths during subsequent cycles of battery operation which determine their performance in long-term run however still remain unclear. By employingvarious electrochemicalcharacterization methods coupled with multiple operando/ex-situ techniques (XRD, XAS, XPS) we studied the detailed phase evolution of few-layered 2H-MoS2. Very interestingly,by means of operando XRD and XAS we found that the MoS2 (de)sodiation mechanism is based on repetitive disintegration and rearrangement of Na2S, Na2S2 and Mo accompanied by amorphization of the sulfur deficient NaxMoSy phase followed by NaxMo3S4 crystallization from an amorphous matrix through electrochemically driven congruent crystallization. Based on or findings we suggest that the high Na-ion diffusion in the NaxMo3S4 and the fast propagation of the interface between the crystallized and the amorphous regions enable very high electrode reaction kinetics. Below some figures which we included in our paper.
MoS2
Within this work we tried to respond to the various scientific questions regarding the mechanism of theelectrochemical reactions ofMoS2electrodes taking place during operation in Na-ion batteries.
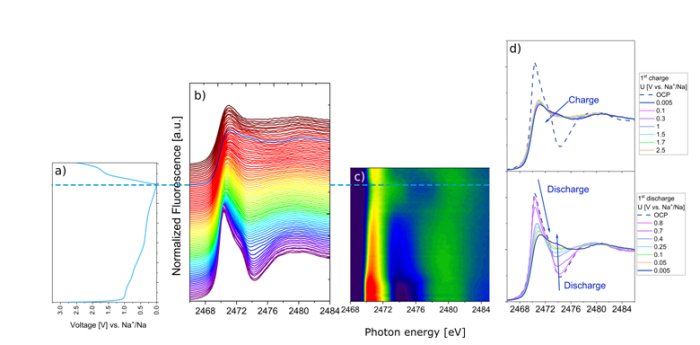
Figure. 1. Operando XAS spectra for the sulfur K-edge registered during the 1st discharge/charge cycle along with voltage characteristics for the measured Na|MoS2 cell. For better visibility, we included the XAS contour map along with selected spectra for particular potentials during the 1st discharge/charge cycle.
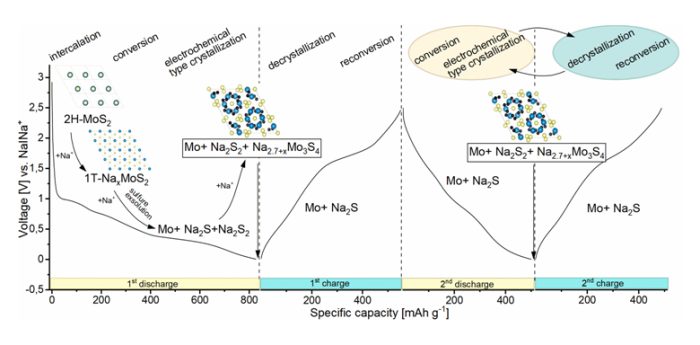
Figure 2. Schematic representation of the phase transitions and processes that take place during the discharge / charge cycles of the Na|MoS2 cell along with its voltage characteristics for the first and second discharge/charge cycles. Intercalation, conversion, sulfur exsolution, and electrochemical-type crystallization were marked during discharge. Conversion/electrochemical type crystallization and decrystalization/reconversion reactions were highlighted as responsible for reactions in subsequent cycles.